Energy-efficient air-conditioning using four cohesive processes
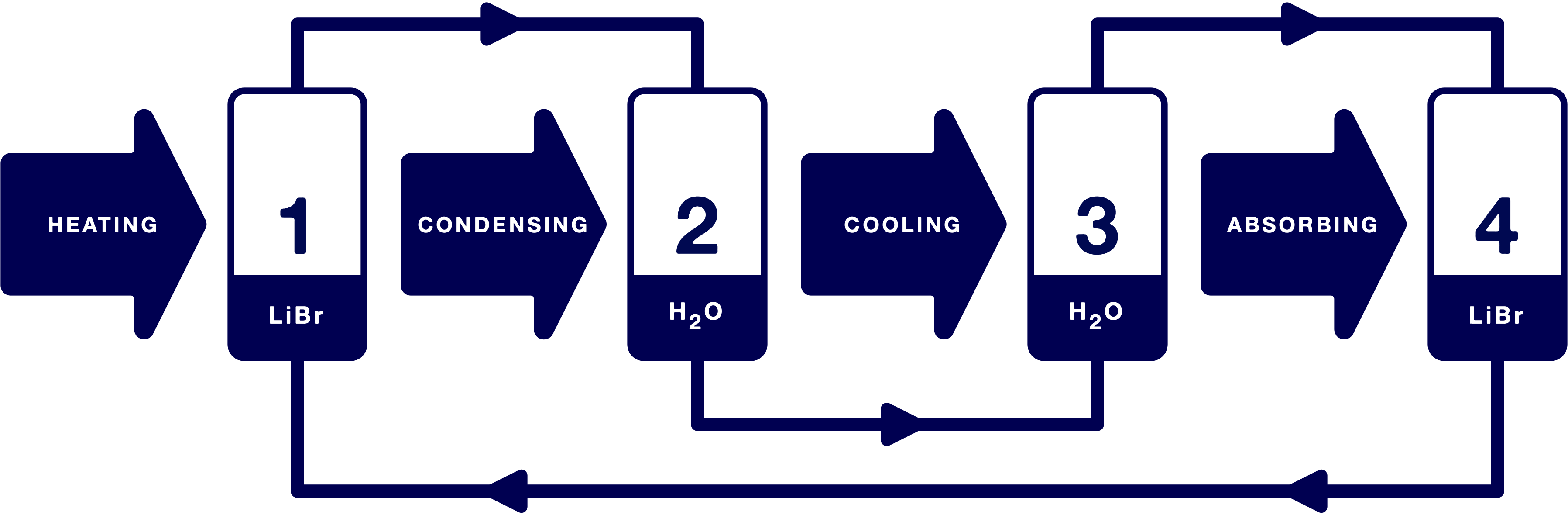
The cooling process is made up of four cohesive processes. These proceed simultaneously in a closed system in a nearly perfect vacuum.
Description of the lithium bromide cooling process – simultaneously in a closed system in a nearly perfect vacuum.
1. An aqueous solution of lithium bromide is heated to the boiling point 
Lithium bromide acts as an absorbent in the system because it has the ability to absorb water extremely well. In process chamber 1, a mixture of lithium bromide and water is heated to the boiling point. Due to the low pressure in the system, the boiling point of the mixture is 60℃. The water vapour is separated out and piped to process chamber 2.
2. There, the water vapour is allowed to condense 
The balance between lithium bromide and water in process chamber 1 is maintained by the injection of a dilute solution from process chamber 4. The water vapour from process chamber 1 is piped to process chamber 2, where the steam is condensed. The condensate, consisting of pure water, is piped to process chamber 3.
3. The refrigeration effect is created by evaporation 
The pressure in process chamber 3 is 8 mbar above absolute vacuum. This causes the condensate from process chamber 3 to evaporate at 5℃. The water acts as a refrigerant in the system. The cooling occurs in process chamber 3 due to the low evaporation temperature.
4. Lithium bromide absorbs the water vapour 
Process chamber 4 contains, like process chamber 1, a mixture of lithium bromide and water. Water vapour from process chamber 3 is sucked into process chamber 4 and is absorbed by the lithium bromide. This dilutes the mixture in process chamber 4. The balance between lithium bromide and water is maintained by the application of a concentrated solution from process chamber 1.